Finding Relief from Rheumatoid Arthritis Helped Three Women Rewrite Their Disease Stories
(BPT) - Content sponsored and provided by Pfizer. All patients partnered with Pfizer to share their experience living with moderate to severe rheumatoid arthritis (RA).
For Ashley - a lifelong athlete, hiker and bird watcher - the turning point of her life with rheumatoid arthritis (RA) came when she broke down on the mound pitching for her softball team and was forced to leave the game. Her joint pain had finally become too much to bear. She wondered if she would be able to maintain the active and outdoor hobbies she enjoys if her symptoms were left unmanaged.
For Lisa - a busy mother and professional on the go - overpowering fatigue, swollen feet and painful fingers had left her wondering how she could possibly keep up. She thought about how RA could impact her full-time job and quality time with family and friends.
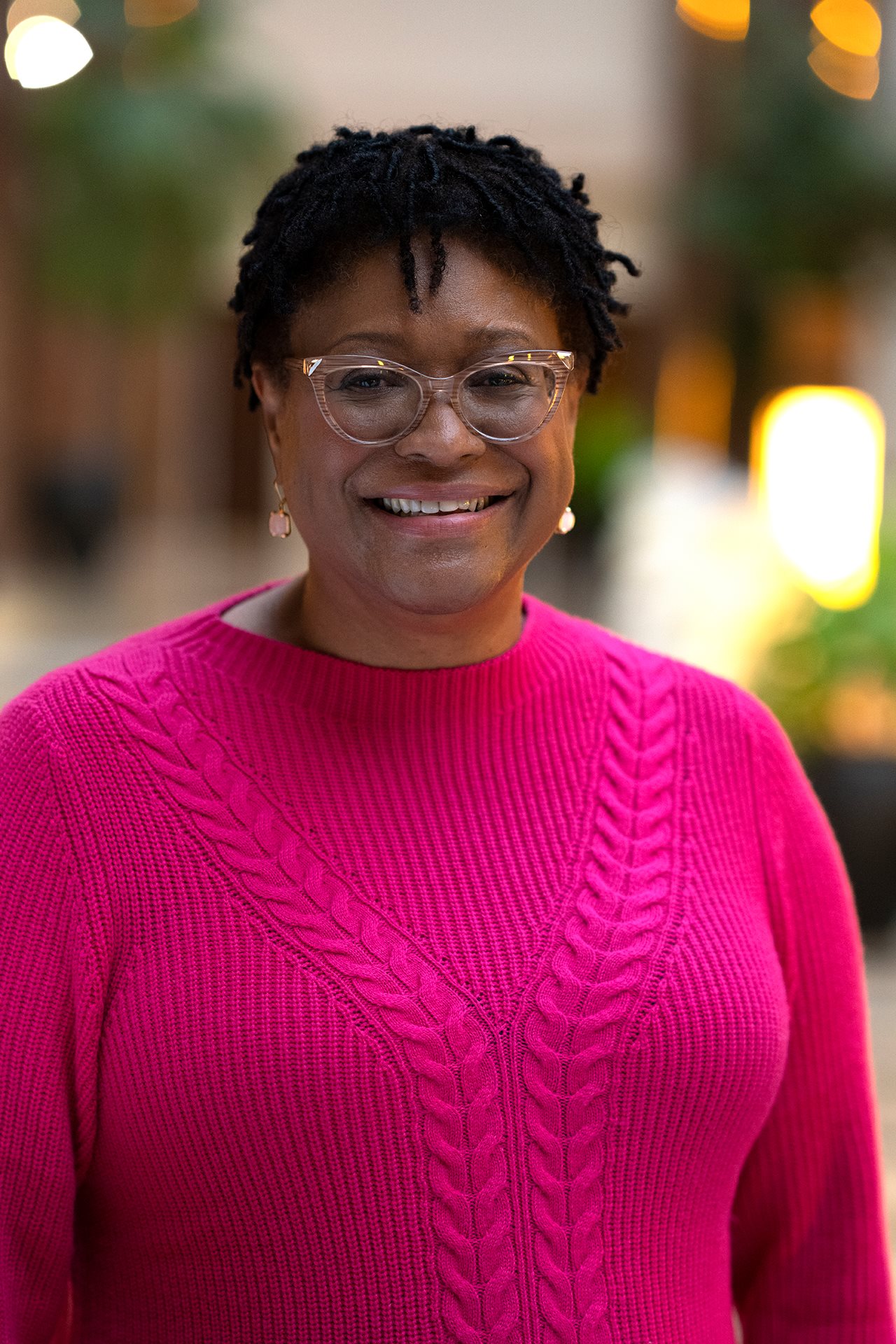
For Alison - a speech therapist with a deep love for the Georgia countryside - her RA diagnosis became real during a conversation with her rheumatologist. When asked, point blank, what she longed to do but couldn't because of her RA, a flood of simple, painful truths surfaced. She wanted to be able to roll over in bed, buckle her seatbelt without a struggle, or shake another person's hand without wincing.
"But when my doctor asked me that," Alison recalled, "I immediately knew what I wanted most was to be able to sit on the floor with my daughter."

Ashley, Lisa and Alison are among the estimated 1.3 million Americans living with rheumatoid arthritis (RA), an inflammatory disease of the joints that's twice as common in women[i] and affects as much as 1% of the world's population[ii].
But that's not where their similarities end.
All three women were living busy, active lifestyles when diagnosed with a moderate to severe form of RA. Each tried a host of different medications - including tumor necrosis factor (TNF) blockers - as part of their determined but unsuccessful effort to meet their treatment goals. And all three women vividly remember the appointment where they aligned with their doctor on their current treatment option.
While researching a medical article she was developing as a freelance writer Ashley had learned about a JAK inhibitor to treat RA. She would ultimately switch doctors in her quest to find a rheumatologist knowledgeable about the available therapies that were more aligned with her treatment goals.
After reading a news article about an FDA-approved RA drug offering symptomatic relief, Lisa mentioned it to her rheumatologist. Coincidentally, he had just returned from a medical conference where he learned about the same medication.
For Alison, her turning point came not long after she posed this question to her doctor: "What is the right amount of pain, stiffness, and swelling to be expected with my diagnosis, and is it wrong to hope for a treatment that could potentially manage my RA symptoms?"
Based on their medical histories and independent consultations with their rheumatologists, Ashley, Lisa and Alison were each informed they were candidates for XELJANZ® (tofacitinib), an oral medication for moderate to severe active RA when one or more medicines called TNF blockers have been used and did not work well or weren't tolerated.
Their rheumatologists reviewed the potential benefits and possible serious side effects of XELJANZ with them and discussed the type of laboratory monitoring that would be needed before and during treatment, including bloodwork. They also explained that XELJANZ has a BOXED WARNING.
XELJANZ can cause serious side effects and can lower your ability to fight infections; don't start XELJANZ if you have an infection. Before and during treatment, your doctor should check for infections like tuberculosis (TB), and do blood tests. Serious, sometimes fatal infections, cancers including lymphoma, and lung cancer, blood clots and serious heart-related events have happened. People 50 or older with heart disease risk factors had an increased risk of death. Tell your doctor if you had a heart attack, other heart problems, stroke, or a history of blood clots.
Read more Important Safety Information and Indication below.
While individual responses to XELJANZ can vary and it may not be right for everyone, Ashley, Lisa and Alison each credit the once-daily oral treatment with helping them manage their RA symptoms.
Ashley says XELJANZ helped improve her symptoms, which led her to go back to grad school, take up reformer Pilates, resume hiking and bird watching, and walk her first full 5K. "While no RA drug is perfect or a cure-all," she says, "XELJANZ has helped me feel better for a longer period of time than other drugs I have tried."

Lisa's symptoms significantly improved after starting XELJANZ, which allowed her to do yoga again, resume family vacations and participate in more activities with her kids. "I became aware that something was changing," she says. "I was not as tired and felt hopeful for the first time in a long time. I started to feel improvement after about two weeks of treatment and continued to feel better as treatment progressed."

Alison credits XELJANZ for helping to relieve her joint pain, allowing her to take family walks again, and for helping with her fatigue, allowing her to make more memories with her family and friends. One of the most special memories, she says, came just before her mother's passing.
"When I was in college, I got into a fight with my mom because I wanted to finish school and she wanted me to stay home so she could take care of me," Alison recalls. "I will forever be thankful for the years my mom was able to see me feeling so well. At her bedside in hospice, I told her to stop worrying about me, that it was okay for her to go. I believe that knowing I was okay gave her peace."

In the end, XELJANZ has had a positive impact on their RA journey. It has allowed all three women to rediscover activities they previously enjoyed. They are now creating new memories with their loved ones.
Learn more about other patients' stories at XELJANZ.com. Not all patients will achieve this response to treatment, individual results may vary.
IMPORTANT SAFETY INFORMATION AND INDICATION
The safety information below applies to all marketed formulations of XELJANZ. Specific risks associated with certain dosing are noted.
Serious infections.XELJANZ can lower the ability of your immune system to fight infections. Do not start taking XELJANZ if you have any kind of infection unless your healthcare provider tells you it is okay. Serious infections have happened in people taking XELJANZ. These serious infections include tuberculosis (TB) and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections. Your healthcare provider should test you for TB before starting and during treatment with XELJANZ. You should not start taking XELJANZ if you have any kind of infection unless your healthcare professional tells you it is okay.
Before and after starting XELJANZ, tell your doctor if you are being treated for an infection, have infections that keep coming back, or have symptoms of an infection, including:
|
|
Increased risk of death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking XELJANZ 5 mg twice daily or XELJANZ 10 mg twice daily.
Cancer.XELJANZ may increase your risk of certain cancers by changing the way your immune system works. Lymphoma and other cancers, including skin cancers, can happen. People taking XELJANZ 5 mg twice daily or XELJANZ 10 mg twice daily have a higher risk of certain cancers including lymphoma and lung cancer, especially if you are a current or past smoker. Tell your healthcare provider if you have ever had any type of cancer.
Higher dose.People with ulcerative colitis taking the higher dose of XELJANZ (10 mg twice daily) or XELJANZ XR (22 mg one time each day) have a higher risk of serious infections, shingles, or skin cancers.
Immune system problem.Some people who have taken XELJANZ with certain other medicines to prevent kidney transplant rejection have had a problem with certain white blood cells growing out of control (Epstein Barr Virus-associated post-transplant lymphoproliferative disorder).
Increased risk of major cardiovascular events such as heart attack, stroke or death in people 50 years of age and older who have at least 1 heart disease (cardiovascular) risk factor and are taking XELJANZ 5 mg twice daily or XELJANZ 10 mg twice daily, especially if you are a current or past smoker.
Get emergency help right away if you have any symptoms of a heart attack or stroke while taking XELJANZ, including:
|
|
Blood clots in the lungs (pulmonary embolism, PE), veins of the legs (deep vein thrombosis, DVT), and arteries (arterial thrombosis)have happened more often in people who are 50 years of age and older and with at least 1 heart disease (cardiovascular) risk factor taking XELJANZ 5 mg twice daily or XELJANZ 10 mg twice daily. Blood clots in the lungs have also happened in people with ulcerative colitis. Some people have died from these blood clots.
- Stop taking XELJANZ and tell your healthcare provider right away if you have any signs and symptoms of blood clots such as sudden shortness of breath, difficulty breathing, chest pain, swelling of a leg or arm, leg pain or tenderness, or red or discolored skin in the leg or arm.
Tears (perforation) in the stomach or intestines.Tell your healthcare provider if you have had diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people taking XELJANZ can get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or methotrexate. Tell your healthcare provider right away if you have fever, stomach-area pain that does not go away, and a change in your bowel habits.
Serious allergic reactions can occur. Stop using XELJANZ and call your healthcare provider right away if you have swelling of your lips, tongue, throat, or get hives.
Changes in certain lab test results.Your doctor should do blood tests to check your white and red blood cells before starting and while you are taking XELJANZ. Your doctor should also check certain liver tests. You should not take XELJANZ if your lymphocyte count, neutrophil count, or red blood cell count is too low or your liver function test levels are too high. Changes in lab test results may cause your healthcare provider to stop your XELJANZ treatment for a time. Your cholesterol levels should be checked 4 to 8 weeks after you start receiving XELJANZ.
Before you use XELJANZ, tell your healthcare provider if you:
- Are being treated for an infection, have an infection that won't go away or keeps coming back, or think you have symptoms of an infection
- Have TB, or have been in close contact with someone with TB, or were born in, lived in, or traveled where there is more risk for getting TB
- Have diabetes, chronic lung disease, HIV, or a weak immune system. People with these conditions have a higher chance for infections
- Live or have lived in certain areas (such as Ohio and Mississippi River Valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections
- Have or have had Hepatitis B or C
- Are a current or past smoker
- Have had any type of cancer
- Have had a heart attack, other heart problems or stroke
- Have had blood clots
- Have liver or kidney problems
- Have any stomach area (abdominal) pain or been diagnosed with diverticulitis or ulcers in your stomach or intestines
- Have recently received or plan to receive a vaccine. People taking XELJANZ should not receive live vaccines but can receive non-live vaccines
- Are pregnant, planning to become pregnant, breastfeeding, or planning to breastfeed. You should not take XELJANZ and breastfeed
- Have had a reaction to tofacitinib or any of the ingredients
- Are taking other medicines, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take any of the following medicines while taking XELJANZ since this may increase your risk of infection:
- tocilizumab (Actemra®)
- etanercept (Enbrel®)
- adalimumab (Humira®)
- infliximab (Remicade®)
- rituximab (Rituxan®)
- abatacept (Orencia®)
- anakinra (Kineret®)
- certolizumab (Cimzia®)
- golimumab (Simponi®)
- ustekinumab (Stelara®)
- secukinumab (Cosentyx®)
- vedolizumab (Entyvio®)
- ixekizumab (Taltz®)
- sarilumab (Kevzara®)
- azathioprine, cyclosporine, or other
immunosuppressive drugs
- Tell your healthcare provider if you are taking medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
What are other possible side effects of XELJANZ/XELJANZ XR?
If you are a carrier of the Hepatitis B or C virus (viruses that affect the liver), the virus may become active while you use XELJANZ. Your healthcare provider may do blood tests before starting and while using treatment with XELJANZ. Tell your healthcare provider if you have any signs of these symptoms: feel very tired, little or no appetite, clay-colored bowel movements, chills, muscle aches, skin rash, skin or eyes look yellow, vomiting, fevers, stomach discomfort, or dark urine.
Common side effects in people with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitisinclude upper respiratory tract infections (common cold, sinus infections), headache, diarrhea, nasal congestion, sore throat, runny nose (nasopharyngitis), high blood pressure (hypertension), and acne.
XELJANZ & Pregnancy
XELJANZ may affect the ability of females to get pregnant. It is not known if this will change after stopping XELJANZ. It is not known if XELJANZ will harm an unborn baby.
You and your healthcare provider should decide if you will take XELJANZ or breastfeed. You should not do both. After you stop your treatment with XELJANZ do not start breastfeeding again until 18 hours after your last dose of XELJANZ or 36 hours after your last dose of XELJANZ XR.
What is XELJANZ/XELJANZ XR?
XELJANZ/XELJANZ XR (tofacitinib) is used to treat adults with:
- Moderately to severely active rheumatoid arthritis when 1 or more medicines called tumor necrosis factor (TNF) blockers have been used, and did not work well or cannot be tolerated
It is not known if XELJANZ/XELJANZ XR is safe and effective in people with Hepatitis B or C.
XELJANZ/XELJANZ XR is not recommended for people with severe liver problems.
It is not known if XELJANZ XR is safe and effective in children.
Please see full Prescribing Information, including BOXED WARNING and Medication Guide.
![]() © 2025 Pfizer Inc. All rights reserved. PP-XEL-USA-10004 April 2025
© 2025 Pfizer Inc. All rights reserved. PP-XEL-USA-10004 April 2025
[i] Rheumatoid arthritis patient education & resources. Information for Rheumatoid Arthritis Patients | Arthritis Foundation. (n.d.). https://www.arthritis.org/rheumatoid-arthritis-patient-education
[ii] Arthritis statistics: What you need to know in 2025. LightSpring Home Care. (2023, August 15). https://lightspringcare.com/blog/arthritis-statistics#.:~:text=Rheumatoid%20Arthritis%20(RA):%20This,1%25%20of%20the%20world's%20population
Source: BrandPoint



















