Eyenovia Announces Progress on Next-Generation User-Filled Optejet Dispensing Device
Eyenovia plans to submit for U.S. device regulatory approval in Q4 of this year, marking a key step toward commercialization
NEW YORK, Feb. 05, 2025 (GLOBE NEWSWIRE) -- Eyenovia, Inc. (NASDAQ: EYEN) (“Eyenovia” or the “Company”), an ophthalmic technology company focused on completing development of its proprietary Optejet® device, today announces recent progress on the development of its user-filled spray dispenser.
“Millions of consumers have difficulty with traditional eye drops, including difficulty with inaccurate administration, discomfort from head tilting, messing up make-up and waste and potential side effects associated with excess drops, all of which could be addressed with the Optejet,” stated Michael Rowe, Chief Executive Officer of Eyenovia. “To that end, we continue to advance development of our novel, user-filled Optejet dispenser and look forward to filing for U.S. regulatory approval for this device in the fourth quarter of this year. We believe the user-filled Optejet can address multi-billion-dollar ophthalmic markets while offering an enhanced user experience.”
Some of the features incorporated into the user-filled Optejet include:
User-Filled Cartridge
The new design includes a sterile disposable cartridge that users can fill using their own, fresh eyedropper bottle. The cartridge is then attached to the reusable base unit and would be capable of dispensing up to 180 metered sprays. Once empty, the cartridge is simply replaced with a new user-filled cartridge.
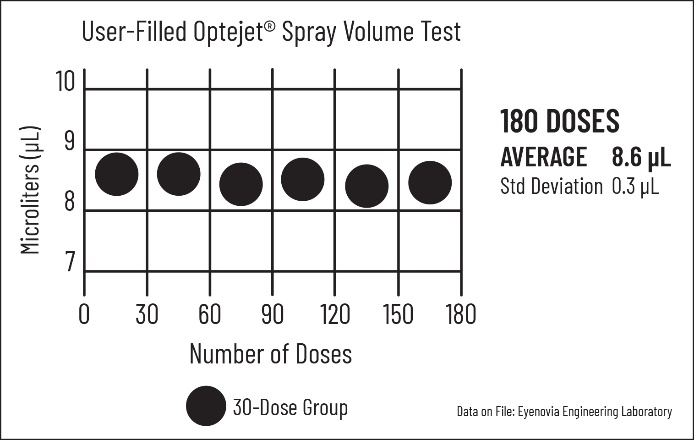
Reliable and Precise Spray
Rigorous testing has resulted in observations of the Optejet’s durable base unit performing over 30,000 sprays, and 98% of the Optejet sprays were between 8-9 microliters (approximately equal to the amount of liquid the eye can hold) over 180 doses, meeting exacting specifications.
Versatile Applications
The user-filled Optejet is designed to work with a variety of topical ophthalmic liquids, such as artificial tears and lens rewetting products, which are expected to generate sales of four billion dollars in the U.S. this year alone.
About Eyenovia, Inc.
Eyenovia, Inc. is an ophthalmic technology company developing its proprietary Optejet topical ophthalmic medication dispensing platform. The Optejet may be especially useful in treatment of chronic front-of-the-eye diseases due to its ease of use, enhanced safety and tolerability, and potential for superior compliance versus standard eye drops. Together, these benefits may combine to produce better treatment options and outcomes for patients and providers. For more information, please visit Eyenovia.com.
Forward Looking Statements
Except for historical information, all the statements, expectations and assumptions contained in this press release are forward-looking statements. Forward-looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions, including those relating to the estimated market opportunities for our platform technology and the regulatory pathway and timing for availability of our products. These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Therefore, actual outcomes and results may, and in some cases are likely to, differ materially from what is expressed or forecasted in the forward-looking statements due to numerous factors discussed from time to time in documents which we file with the U.S. Securities and Exchange Commission.
In addition, such statements could be affected by risks and uncertainties related to, among other things: the potential advantages of our products and platform technology; the regulatory pathway that would apply to our products; our estimates regarding the potential market opportunity for our products; reliance on third parties to develop and commercialize our products; the ability of us and our partners to timely develop, implement and maintain manufacturing, commercialization and marketing capabilities and strategies for our products; intellectual property risks; changes in legal, regulatory, legislative and geopolitical environments in the markets in which we operate and the impact of these changes on our ability to obtain and maintain regulatory approval for our products and product candidates; our competitive position; our ability to raise additional funds and to make payments on our debt obligations as and when necessary; and our ability to pursue strategic alternatives.
Any forward-looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, Eyenovia does not undertake any obligation to update any forward-looking statements.
Eyenovia Contact:
Eyenovia, Inc.
Norbert Lowe
Sr. Vice President, Commercial Operations
admin@eyenovia.com
Eyenovia Investor Contact:
Eric Ribner
LifeSci Advisors, LLC
eric@lifesciadvisors.com
(646) 751-4363
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/405b239a-8b0f-4d1f-a650-aa6bf45d84e9

© 2025 GlobeNewswire, Inc. All Rights Reserved.



















