Corvus Pharmaceuticals Announces Data from Cohorts 1-3 of Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
Data continue to demonstrate favorable safety and efficacy profile, including earlier and deeper responses in cohort 3 compared to cohorts 1-2
Data to be presented in an oral session and poster at the Society for Investigative Dermatology 2025 Annual Meeting
Company to discuss data today on its first quarter 2025 business update conference call and webcast at 4:30 pm ET / 1:30 pm PT
SOUTH SAN FRANCISCO, Calif., May 08, 2025 (GLOBE NEWSWIRE) -- Corvus Pharmaceuticals, Inc. (NASDAQ: CRVS), a clinical-stage biopharmaceutical company, today announced new interim data from the randomized, double-blind, placebo-controlled Phase 1 clinical trial evaluating soquelitinib in patients with moderate to severe atopic dermatitis. The data demonstrated a favorable safety and efficacy profile, including earlier and deeper responses in cohort 3 (200 mg twice per day, total daily dose 400 mg) compared to cohorts 1 and 2 (100 mg twice per day and 200 mg once per day, total daily dose 200 mg). Overall, all three cohorts showed significant responses in the soquelitinib treatment groups compared to placebo for clinically significant endpoints of EASI (Eczema Area and Severity Index) 75 and IGA (Investigator Global Assessment) 0 or 1.
“We continue to be encouraged by the results from our Phase 1 trial of soquelitinib in patients with atopic dermatitis, which show a favorable safety and efficacy profile with a convenient oral tablet,” said Richard A. Miller, M.D., co-founder, president and chief executive officer of Corvus. “We believe the results are particularly exciting given the relatively short treatment duration of 28 days and durable post-treatment results, both of which we associate with the novel mechanism of action provided by ITK inhibition. This is supported by biomarker studies from the trial, which show evidence of multiple effects derived from the selective blockade of ITK including the reduction of various inflammatory cytokines and the potential induction of T regulatory cells, further amplifying the disease modifying effects of soquelitinib. Based on these results, we have amended the trial protocol to evaluate an additional 24 patients at the 200 mg twice per day dose, randomized one-to-one with placebo for an extended treatment period of 8 weeks. This amendment gives us the opportunity to evaluate the potential for even stronger efficacy with longer treatment duration. This additional experience is intended to help optimize the design of a Phase 2 trial, which we are on track to initiate before the end of the year.”
Soquelitinib Interim Data from the Atopic Dermatitis Phase 1 Clinical Trial
As of May 6, 2025, enrollment into cohorts 1, 2 and 3 has been completed for a total of 48 patients. The data covers 32 patients receiving soquelitinib and 12 placebos with 28-day follow-up, and four additional patients receiving soquelitinib with 15-day follow-up from cohort 3. These four patients have not yet completed the 28-day treatment course.
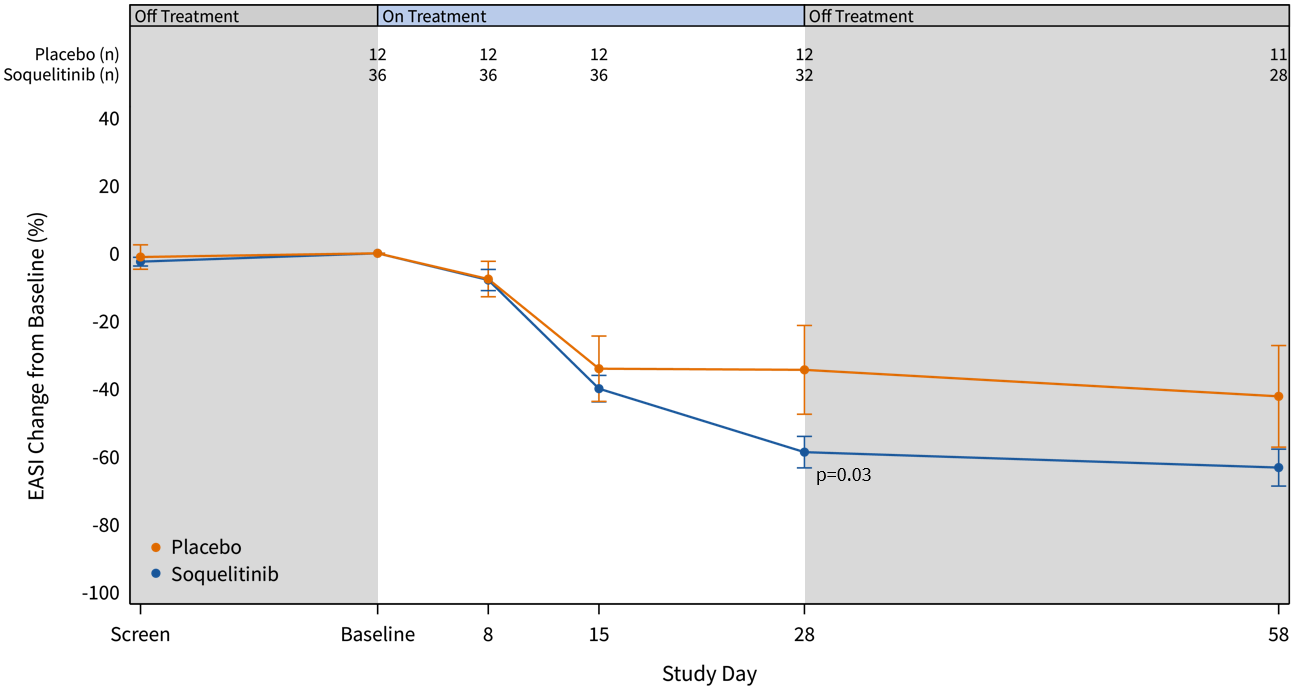
Baseline patient characteristics are shown below in Table 1. Patients enrolled in cohort 3 had more severe disease with higher mean baseline EASI scores compared to cohorts 1 and 2. A higher proportion also failed prior systemic therapies. Across all cohorts, the mean EASI scores are 22.3 and 21.2 for active and placebo, respectively. Placebo (n=12) and active (n=36) treatment groups were well-balanced with regard to baseline characteristics.
Table 1: Patient Characteristics
The percent reduction in mean EASI scores at 28 days for the combined cohort 1 and 2 group was 54.6% for patients receiving soquelitinib and 30.6% for patients receiving placebo. In cohort 3, the percent reduction in mean EASI score at 28 days was 71.1% for patients receiving soquelitinib and 42.1% for patients receiving placebo.
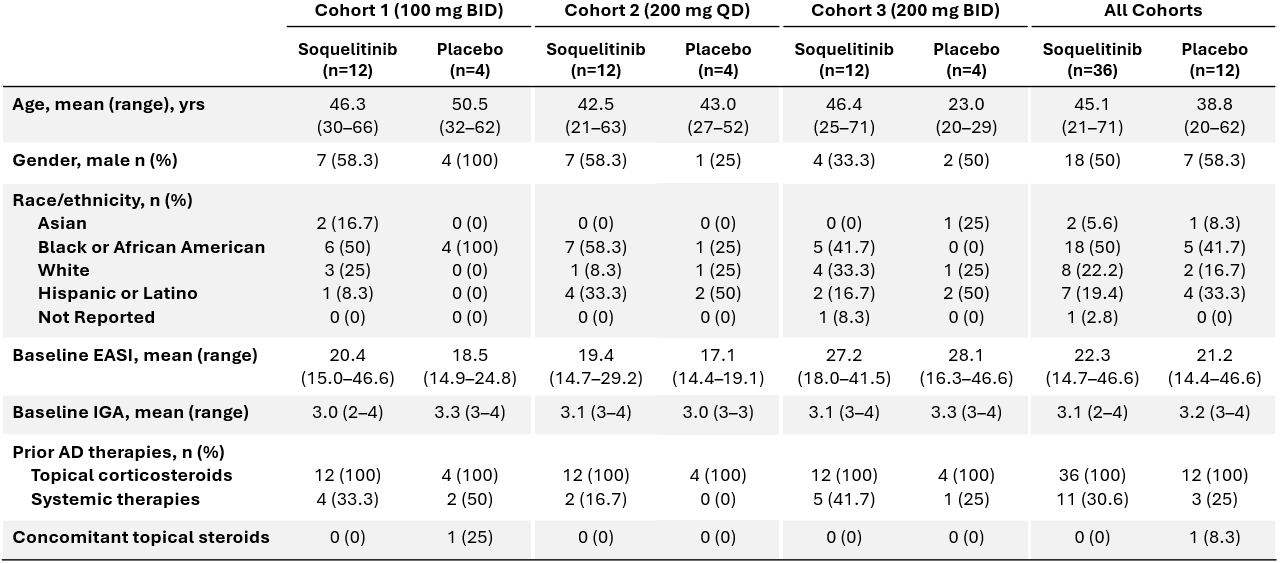
Figure 1 below shows the percent of patients that achieved IGA 0 or 1 or EASI 75 at day 28 of treatment. The placebo patients from cohort 1 (n=4), cohort 2 (n=4) and cohort 3 (n=4) are combined, with no placebo patients achieving IGA 0 or 1 or EASI 75. IGA 0 or 1 and EASI 75 have been determined by the U.S. Food and Drug Administration (FDA) to be clinically meaningful and approvable endpoints and have been the endpoints used in clinical trials for other FDA approved treatments for atopic dermatitis.
Figure 1: Percent Patients Achieving Endpoints IGA 0 or 1, EASI 75 at Day 28 of Treatment
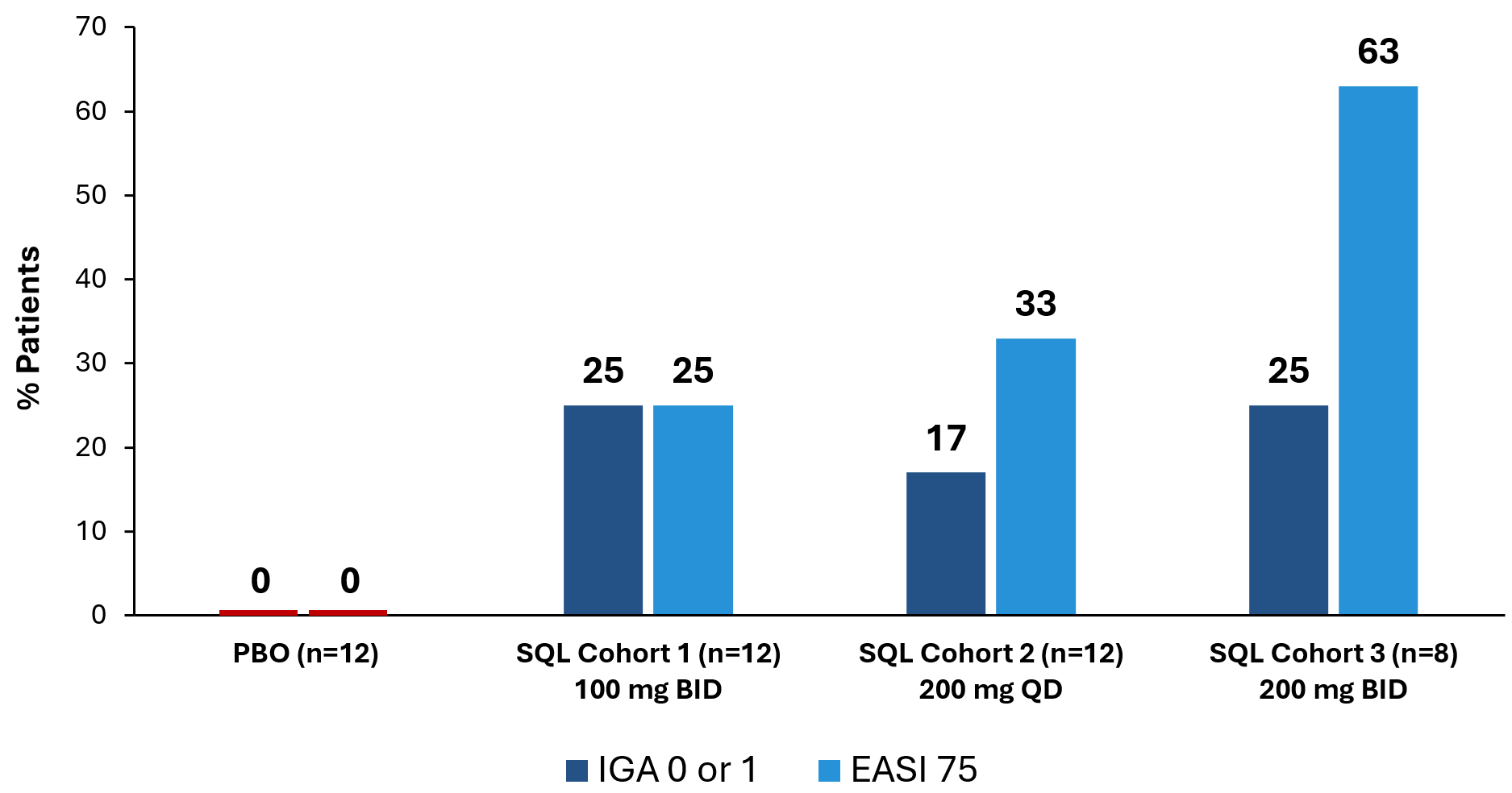
The graphs below (Figures 2 and 3) show the kinetics of response for each of the cohorts and for the combined cohorts 1, 2 and 3. The placebo patients (n=12) are combined in both graphs. Separation of the curves for patients receiving active drug began at day 15 and increased by day 28 for cohorts 1 and 2. Cohort 3 patients experienced earlier and deeper separation from placebo starting by day 8. The combined soquelitinib treatment group is significantly superior to placebo at day 28, p=0.03.
Figure 2: Percent Reduction in Mean EASI Score for Cohorts 1, 2 and 3. Mean percent change in EASI score over time is shown. Treatment beginning is designated “Baseline” and days post-baseline are shown. Screening to baseline data is shown and demonstrates relative disease stability. The study blinding remains in effect for the entire 58-day period. Numbers at the top of the graphs indicate numbers of patients evaluated at the various time points.
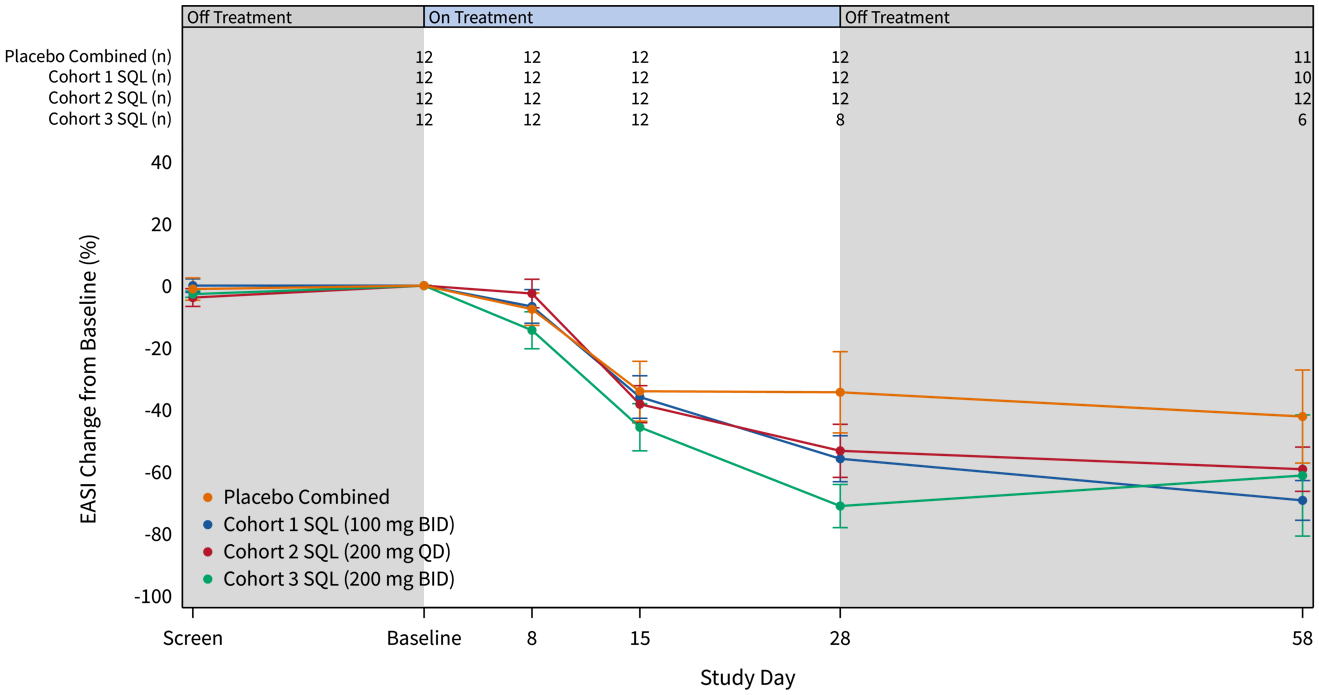
Figure 3: Percent Reduction in Mean EASI Score for Combined Cohorts 1, 2 and 3.The data is displayed below with cohorts combined. Four active patients in cohort 3 have not yet reached day 28 of treatment.
Safety Data
Soquelitinib was well tolerated, with no dose limiting toxicities (DLTs) and no clinically significant laboratory abnormalities observed in any of the cohorts. No interruption of drug dosing was seen in any of the cohorts. Grade 1/2 adverse events (treatment related and unrelated) were seen in 33.3% of patients receiving soquelitinib and 25% receiving placebo. Only one treatment related adverse event of grade 1 nausea was reported with soquelitinib treatment.
Serum Cytokine and Other Biomarker Studies
As reported previously, relationships between reductions in certain cytokines with improvement in EASI scores were observed. Reductions in serum cytokine levels were seen for IL-5, IL-9, IL-17, IL-31, IL-33, TSLP and TARC. Differences between responding and non-responding patients were found, while no such relationships were seen in the placebo group. Increasing trends were seen in numbers of circulating T regulatory cells, consistent with the presumed mechanism of action of soquelitinib.
Soquelitinib Atopic Dermatitis Phase 1 Clinical Trial Design and Protocol Update
The randomized, double-blind, placebo-controlled Phase 1 clinical trial was designed to enroll 64 patients with moderate to severe atopic dermatitis that previously failed one prior topical or systemic therapy. Patients were planned to be enrolled into one of four dosing cohorts in a 3:1 ratio (12 active and four placebo) to receive either soquelitinib or placebo. The cohorts are sequentially enrolled and will examine 100 mg orally twice per day, 200 mg orally once per day, 200 mg orally twice per day and 400 mg orally once per day. Patients are treated for 28 days and are then followed for an additional 30 days with no therapy. The Company amended the clinical trial protocol to replace cohort 4 (400 mg once per day) with 24 patients randomized 1:1 between active and placebo. Treatment for this group will be extended to 8 weeks with additional 30-day follow-up with no treatment. The dose level for this group is planned to be the same as cohort 3 – 200 mg orally twice per day.
These doses were selected based on the Company’s prior experience evaluating soquelitinib in T cell lymphoma patients. The doses in the atopic dermatitis trial studied in cohorts 1 and 2 are lower than the 200 mg orally twice a day dosing regimen (same dose as cohort 3 of the atopic dermatitis trial), which is the level that has been shown to provide complete ITK occupancy and that is being evaluated in the Company’s ongoing registrational Phase 3 clinical trial of soquelitinib in peripheral T cell lymphoma.
The primary endpoints include safety and tolerability. Efficacy, measured by improvement in EASI score and IGA, are secondary endpoints. Reduction in itch and various cytokine biomarkers are exploratory endpoints. EASI scores are also evaluated by the percent of patients that achieve a specified percent reduction in EASI score – EASI 50 for patients that achieved a 50% reduction; EASI 75 for a 75% reduction; and EASI 90 for a 90% reduction. Corvus and a data monitoring committee monitor the data from the trial as the trial progresses.
Presentation at Society for Investigative Dermatology 2025 Annual Meeting
The data from the Phase 1 clinical trial of soquelitinib for atopic dermatitis will be presented by Albert S. Chiou, M.D., MBA, Clinical Associate Professor, Dermatology and Director of Clinical Research in the Department of Dermatology at Stanford University Medical Center. Dr. Chiou’s clinical focus is in medical dermatology and he conducts clinical trials investigating new therapies for inflammatory and genetic skin disease, including atopic dermatitis. The details of Dr. Chiou’s presentations are as follows:
- Abstract Title: Soquelitinib, a selective ITK inhibitor demonstrates activity in atopic dermatitis phase 1 clinical trial by a novel mechanism of action
- Abstract #: 0437
- Poster Presentation Date and Time: May 8, 2025 from 4:30 – 6:00 pm PT
- Oral Presentation Date and Time: May 10, 2025 from 9:50 – 10:00 am PT
Upcoming Presentation and Webcast
Dr. Miller will present details on the new data from the soquelitinib Phase 1 clinical trial during the Company’s first quarter 2025 business update conference call and webcast today, Thursday, May 8, 2025 at 4:30 pm ET / 1:30 pm PT. The conference call can be accessed by dialing 1-800-717-1738 (toll-free domestic) or 1-646-307-1865 (international) or by clicking on this link for instant telephone access to the event. The live webcast, which will include presentation slides, may be accessed via the investor relations section of the Corvus website. A replay of the webcast will be available on Corvus’ website for 60 days.
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company’s lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com or follow the Company on LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements related to the potential of the Company’s product candidates including soquelitinib and the interim results from the Phase 1 trial of soquelitinib in patients with atopic dermatitis, the design and timing of initiation of a Phase 2 trial, the advancement of ITK inhibition and the opportunities it provides, and continued advancement of the Company’s clinical pipeline. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Quarterly Report on Form 10-Q for the first quarter ended March 31, 2025, filed with the Securities and Exchange Commission on or about the date hereof, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of its product candidates; the accuracy of the Company’s estimates relating to its ability to initiate and/or complete preclinical studies and clinical trials and release data from such studies and clinical trials; the results of preclinical studies and interim data from clinical trials not being predictive of future results; the Company’s ability to enroll sufficient numbers of patients in its clinical trials; the unpredictability of the regulatory process; regulatory developments in the United States and foreign countries; the costs of clinical trials may exceed expectations; and the Company’s ability to raise additional capital. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and the timing of events and circumstances and actual results could differ materially from those projected in the forward-looking statements. Accordingly, you should not place undue reliance on these forward-looking statements. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
INVESTOR CONTACT:
Leiv Lea
Chief Financial Officer
Corvus Pharmaceuticals, Inc.
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Sheryl Seapy
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/08905c00-1401-44f7-a727-57f812163f24
https://www.globenewswire.com/NewsRoom/AttachmentNg/f00ab519-b9f4-45cd-9a86-2164be1c8931
https://www.globenewswire.com/NewsRoom/AttachmentNg/d0831187-c4ea-4439-b2d1-92922ebedd96
https://www.globenewswire.com/NewsRoom/AttachmentNg/df64aeaa-33f0-4acd-a159-da50d7fc7de9

© 2025 GlobeNewswire, Inc. All Rights Reserved.
BREAKING NEWS: Crown Equity Holdings, Inc. Announces Partnership


















