Immuron - FY25 global sales exceed projection, up 49% on prior year
Sales Highlights (unaudited):
| Global |
|
| Australia |
|
| North America |
|
MELBOURNE, Australia, July 17, 2025 (GLOBE NEWSWIRE) -- Immuron Limited (ASX: IMC; NASDAQ: IMRN), an Australian based and globally integrated biopharmaceutical company, is pleased to announce continued sales growth (unaudited) of Travelan®, an over-the-counter immune supplement that targets pathogenic bacteria and the toxins they produce in the gastrointestinal (GI) tract.
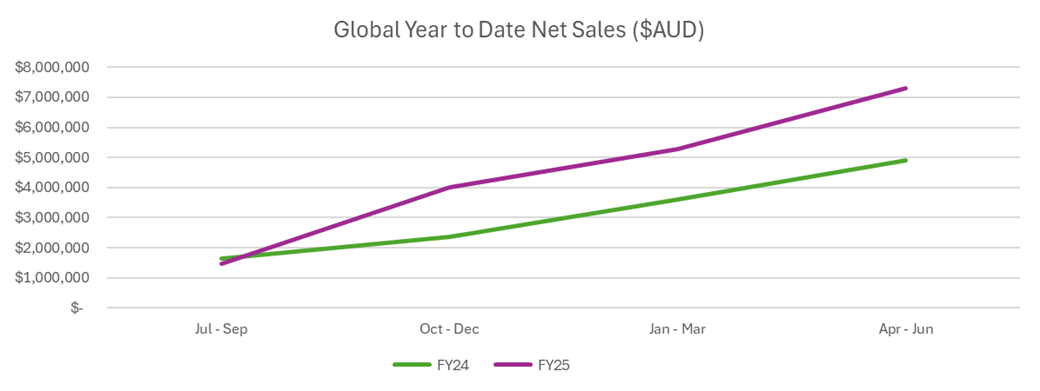
Flavio Palumbo, Chief Commercial Officer, said, “Immuron has exceeded sales projections and achieved record FY25 sales of A$7.3 million, with growth just shy of 50% on last year. In FY25, we set out to make Travelan the must-have travel essential for consumers and retailers. This is a fantastic result, supporting our clear growth strategy. Australia, on the back of increased consumer engagement and a clear pharmacy visibility program, achieved annual sales of A$5.2 million, growth of 40% on last year. Travelan is now one of the fastest growing brands in pharmacy in Australia. Our North American business continues to produce record results with strong USA sales to Amazon with improved, more targeted communication focused on the benefits of Travelan driving more users into the brand. This, together with establishing good distribution of Travelan in Canada, resulted in North American sales of A$2 million, growth of 76% on last year. We have achieved record Travelan sales in all markets. In FY26, we will invest to grow in North America while continuing the momentum in Australia.”
This release has been authorised by the directors of Immuron Limited.
Steven Lydeamore
Chief Executive Officer
steve@immuron.com
About Travelan®
Travelan® is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea, a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a purified tablet preparation of hyper-immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing bacteria and prevent colonization and the pathology associated with traveler’s diarrhea. In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Traveler’s Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product (NPN 80046016) and is indicated to reduce the risk of Traveler’s Diarrhea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection.
About Traveler’s diarrhea
Traveler’s Diarrhea is a gastrointestinal infection with symptoms that include loose, watery (and occasionally bloody) stools, abdominal cramping, bloating, and fever, Enteropathogenic bacteria are responsible for most cases, with enterotoxigenic Escherichia coli (ETEC) playing a dominant causative role.
About Immuron
Immuron Limited (ASX: IMC, NASDAQ: IMRN) is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of inflammatory mediated and infectious diseases.
For more information visit: https://www.immuron.com.au
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law.
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/d24a5b18-311e-4dc1-b624-1162a4d7acc9

© 2025 GlobeNewswire, Inc. All Rights Reserved.



















