Medtronic announces FDA clearance of Hugo™ robotic-assisted surgery system for urologic surgical procedures
Medtronic announces FDA clearance of Hugo™ robotic-assisted surgery system for urologic surgical procedures |
| [03-December-2025] |
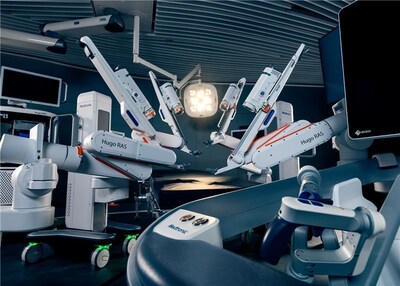
Hugo RAS system brings choice to the U.S., coupled with the full suite of Medtronic surgical offerings, ultimately creating a connected and integrated operating room today and into the future. GALWAY, Ireland, Dec. 3, 2025 /PRNewswire/ -- Medtronic (NYSE: MDT), a global leader in surgical innovation, today announced the U.S. Food and Drug Administration (FDA) has cleared the Hugo™ robotic-assisted surgery (RAS) system for use in urologic surgical procedures. The Hugo RAS system's clearance brings a versatile robotic-assisted platform to U.S. surgeons and health systems seeking to expand soft-tissue robotic surgery programs and access to minimally invasive care. The U.S. leads the world in robotic surgery adoption, yet hospitals continue to face challenges in capacity and access. The introduction of the Hugo RAS system in the U.S. helps address this gap, offering an innovative approach to robotic-assisted surgery from the global leader in surgery and bringing choice to hospitals and surgeons across the country. "This is an incredibly exciting day for healthcare in the United States. FDA clearance of the Hugo RAS system means there is now choice for hospitals looking to expand their robotic programs and increases access for patients," said Rajit Kamal, vice president and general manager of Robotic Surgical Technologies within the Surgical business of Medtronic. "As we begin our purposeful launch of the Hugo RAS system in the U.S., our focus is on building a strong foundation with leading hospitals through our differentiated approach to partnership, rooted in our enduring commitment to provide an excellent customer experience and enable surgical teams to deliver the best possible outcomes for their patients." Thoughtfully designed with input from surgeons and hospital administrators to shape the future of surgery, the Hugo RAS system includes three main differentiators:
"The Hugo RAS system represents a new and exciting approach to robotic-assisted surgery," said Dr. James Porter, a urologic surgeon and chief medical officer for Robotic Surgical Technologies and Digital Technologies within the Surgical business at Medtronic. "We're excited for surgical teams in the U.S. to experience the differentiated technology and partnership from Medtronic, which supports them at every stage of their robotic surgical journey." With FDA clearance, the Hugo RAS system is indicated for use in minimally invasive urologic surgical procedures including prostatectomy, nephrectomy, and cystectomy — common procedures that account for about 230,000 surgeries per year in the U.S. The Expand URO investigational device exemption clinical study — the largest ever completed for multi-port robotic-assisted urological surgery in the U.S. — demonstrated that the Hugo RAS system met primary safety and effectiveness endpoints in urologic surgical procedures, with outcomes that are consistent with published literature. Outside the U.S., the Hugo RAS system has been used in tens of thousands of urologic, gynecologic, and general surgery procedures in more than 30 countries across 5 continents. Medtronic intends to expand the Hugo RAS system's use in the U.S. to additional surgical specialties over time, with indications for general and gynecologic surgical procedures expected to follow the initial urology clearance. The introduction of the Hugo RAS system in the U.S. builds on Medtronic's broad surgical offering, including the Touch Surgery ecosystem, to deliver a connected, integrated operating room — today and in the future. For more information, visit medtronic.com/hugoishere. †The Medtronic Touch Surgery™ ecosystem is not intended to direct surgery, or aid in diagnosis or treatment of a disease or condition. Performance Insights are available for select procedures, instruments, and anatomy. About Medtronic Any forward-looking statements are subject to risks and uncertainties such as those described in Medtronic's periodic reports on file with the Securities and Exchange Commission. Actual results may differ materially from anticipated results. Contacts: Ryan Weispfenning
SOURCE Medtronic plc | ||
Company Codes: NYSE:MDT |