BD Receives FDA 510(k) Clearance for EnCor EnCompass™ Breast Biopsy and Tissue Removal System
BD Receives FDA 510(k) Clearance for EnCor EnCompass™ Breast Biopsy and Tissue Removal System |
| [15-January-2026] |
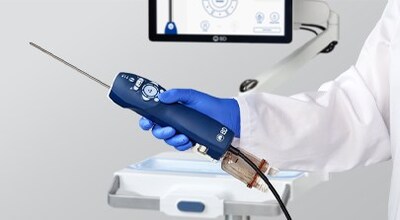
State-of-the-art, multi-modality breast biopsy system slated to be in the market in early 2026 FRANKLIN LAKES, N.J., Jan. 15, 2026 /PRNewswire/ -- BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the EnCor EnCompass™ Breast Biopsy and Tissue Removal System, a state-of-the-art multi-modality breast biopsy system designed to provide clinicians with flexibility across breast imaging modalities in the diagnosis of breast disease. "This milestone for our new breast biopsy system marks a meaningful advancement in breast health, playing a critical role in aiding the early detection and diagnosis of breast disease," said Rima Alameddine, worldwide president, Peripheral Intervention at BD. "This innovation underscores our commitment to partnering with clinical leaders to deliver patient-centered solutions. Guided by our vision to transform breast health, we remain focused on developing technologies that empower providers and inspire confidence in care." The EnCor EnCompass™ Biopsy System is designed to streamline the breast biopsy experience by enabling clinicians to perform procedures across a range of breast imaging platforms using one integrated system. The system, which is expected to enter the market in early 2026, offers a combination of advanced features and user-focused design to support procedural efficiency. "The FDA clearance of the EnCor EnCompass™ Biopsy System demonstrates our ongoing focus on addressing the evolving needs of clinicians and patients in breast health," said Stacie Watson, vice president and general manager of the Oncology Platform at BD Interventional–Peripheral Intervention. "This multi-modality platform is engineered to provide flexibility, control, and ease of use, with features designed to support both clinician confidence and the patient experience." Key features of the EnCor EnCompass™ Biopsy System include:
"Our goal is always to provide the best possible care for patients while maintaining efficiency, accuracy, and safety," said Dr. Shadi Aminololama-Shakeri, M.D., Chief of Breast Radiology at UC Davis. "The EnCor EnCompass™ Biopsy System combines multi-modality capability and enhanced control into one platform that supports intraprocedural customization and helps streamline the biopsy process." The clearance of the EnCor EnCompass™ Biopsy System expands BD's breast health portfolio and reflects the Company's ongoing commitment to advancing technologies that support early detection and diagnosis. About BD
Indications for Use: The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is indicated to acquire breast tissue for histologic examination with partial or complete removal of the abnormality. For more information, please consult the product labels and inserts for any indication, contraindications, hazards, precautions BD, the BD Logo, Advancing the world of health and EnCor EnCompass are trademarks of Becton, Dickinson and Company or its affiliates. © 2026 BD. All Rights Reserved. BD-165500
SOURCE BD (Becton, Dickinson and Company) | ||||||||||||||||||
Company Codes: NYSE:BDX |